Abstract
Introduction: CD25, also known as interleukin-2 receptor α chain (IL-2Rα), forms a heterotrimer with IL-2Rβ and common γ chain, and mediates IL-2 signaling. IL-2 signaling is essential for T- and NK-cell survival and proliferation. Additionally, CD25 is required for development of thymic regulatory T cells (Treg) and their suppressive function. Interestingly, CD25 is upregulated in a variety of lymphoid and myeloid malignancies, which do not depend on IL-2 signaling, suggesting IL-2-independent roles of CD25. Here, we seek to investigate whether CD25 has previously unrecognized functions in T cells and T-cell lymphomas.
Results: Our previous study in B cells has discovered the cytoplasmic tail of CD25 as an essential structural element for site-specific recruitment of inhibitory phosphatases. CD25-mediated shuttling of inhibitory phosphatases balanced strength of oncogenic B-cell receptor (BCR)-signaling and was essential for cell-survival in B-cell malignancies. Interestingly, we found that CD25 expressed on B cells was monomeric and showed dynamic recruitment to BCR or its oncogenic mimics within lipid rafts. Studying gene expression data across different hematopoietic lineages, we found CD25 was strongly induced in T cell receptor (TCR)-activated T cells, as well as in T-cell malignancies that harbor oncogenic TCR-mimics, such as PTCL.
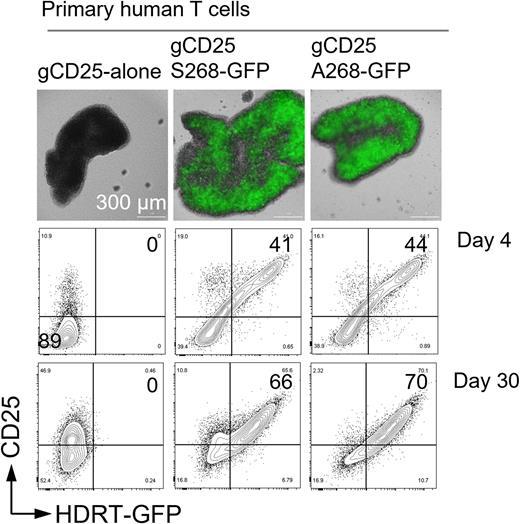
Our mechanistic studies discovered that the serine residue 268 (S268) in the cytoplasmic tail of CD25 was a principal substrate of protein kinase C (PKC), and that S268 phosphorylation by PKC was critical for cell surface expression of CD25. The proximity-based labeling assays (Bio-ID) revealed that CD25-S268 but not its phospho-dead mutant recruits PKC and its adapter RACK1, which scaffold the inhibitory phosphatases SHIP1 and SHP1 for site-specific activation in lipid rafts. CRISPR-mediated knock-in of CD25-S268A that abrogates the formation of inhibitory phosphatase complexes in primary human T cells enabled us to specifically interrogate the functional relevance of CD25-S268 in the regulation of TCR signaling. By using single stranded homology-directed repair templates (ssHDRT) that contain a Cas9 shuttling sequence, we were able to achieve 70% knock-in efficiency in primary human T cells (Figure 1). Signaling studies with these cells revealed that S268A mutation significantly augmented TCR signaling strength. Additionally, S268A mutation resulted in acute inactivation of inhibitory phosphatases including SHP1. Consistent with these results, CD25 shuttling to TCR complex was promptly induced upon TCR stimulation and was mitigated by S268 mutation. Furthermore, immunophenotyping showed that loss of S268 phosphorylation resulted in increased expression of T-cell activation markers such as CD44 and CD69. Based on the ex vivo results, we generated a transgenic mouse model with germline knock-in of CD25-S268A, which would allow us to study S268-specific roles in CD25-mediated feedback regulation of TCR and oncogenic signaling in vivo.
Conclusions: The role of CD25 in mediating IL-2 signaling has been extensively studied. However, increasing evidence suggests that CD25 plays a more sophisticated role in TCR signaling and T-lymphomagenesis. Here we discovered a previously unrecognized function of CD25 as a feedback regulator of TCR signaling. TCR activation or oncogenic TCR-mimics induce CD25-mediated recruitment of inhibitory phosphatases in lipid rafts to mitigate TCR signaling or curb excessive oncogenic signaling.
Figure 1. CRISPR-mediated knock-out of CD25 alone, or followed by knock-in of CD25-S268-GFP or CD25-A268-GFP, in primary human T cells. The proportion of CD25-expressing GFP+ cells are analyzed by flow cytometry on day 4 and day 30 post CRISPR.
Disclosures
Marson:Vertex: Other: Speaking and/or advising fees; Juno Therapeutics: Other: Speaking and/or advising fees, Research Funding; GlaxoSmithKline: Research Funding; Survey Genomics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-founder; Anthem: Research Funding; Bernstein: Other: Speaking and/or advising fees; Rupert Case Management: Other: Speaking and/or advising fees; ALDA: Other: Speaking and/or advising fees; AlphaSights: Other: Speaking and/or advising fees; Arsenal Biosciences: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Compensated co-founder; Gilead: Research Funding; PACT Pharma: Current equity holder in private company, Other: Speaking and/or advising fees; Merck: Divested equity in a private or publicly-traded company in the past 24 months, Other: Speaking and/or advising fees; Sanofi: Research Funding; NewLimit: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Spotlight Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Compensated co-founder; Offline Ventures: Other: Investor, informal advisor; Amgen: Other: Speaking and/or advising fees; Epinomics: Research Funding; Trizell: Other: Speaking and/or advising fees; Genentech: Other: Speaking and/or advising fees; EPIQ: Other: Client; 23andMe: Other: Speaking and/or advising fees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal